The Role of Genetics in DMD
Genetic Mutations in DMD
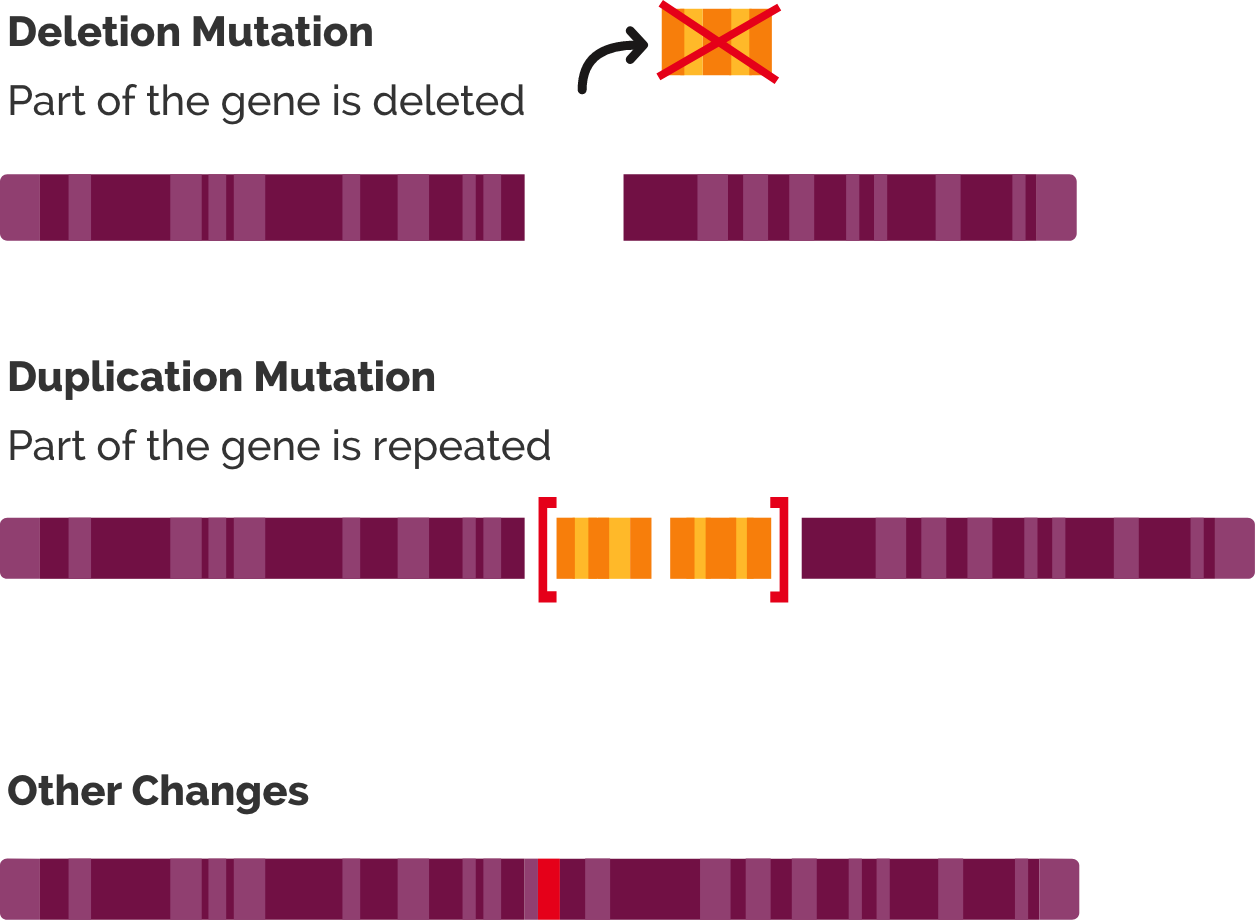
Several types of genetic mutations can cause DMD1,2:
- Large deletions: One or more exons are missing from the DMD gene, which codes for the dystrophin protein
- Large duplications: One or more exons have extra copies in the DMD gene
- Other changes: Small changes, such as deletions or changes in a single nucleotide in the gene
The most common mutation in people with DMD is a deletion of one or more exons.2 These deletions cause frameshift mutations, which leads to the exons following the deletion being “out of frame.” This disrupts translation of the gene to produce a working dystrophin protein.2
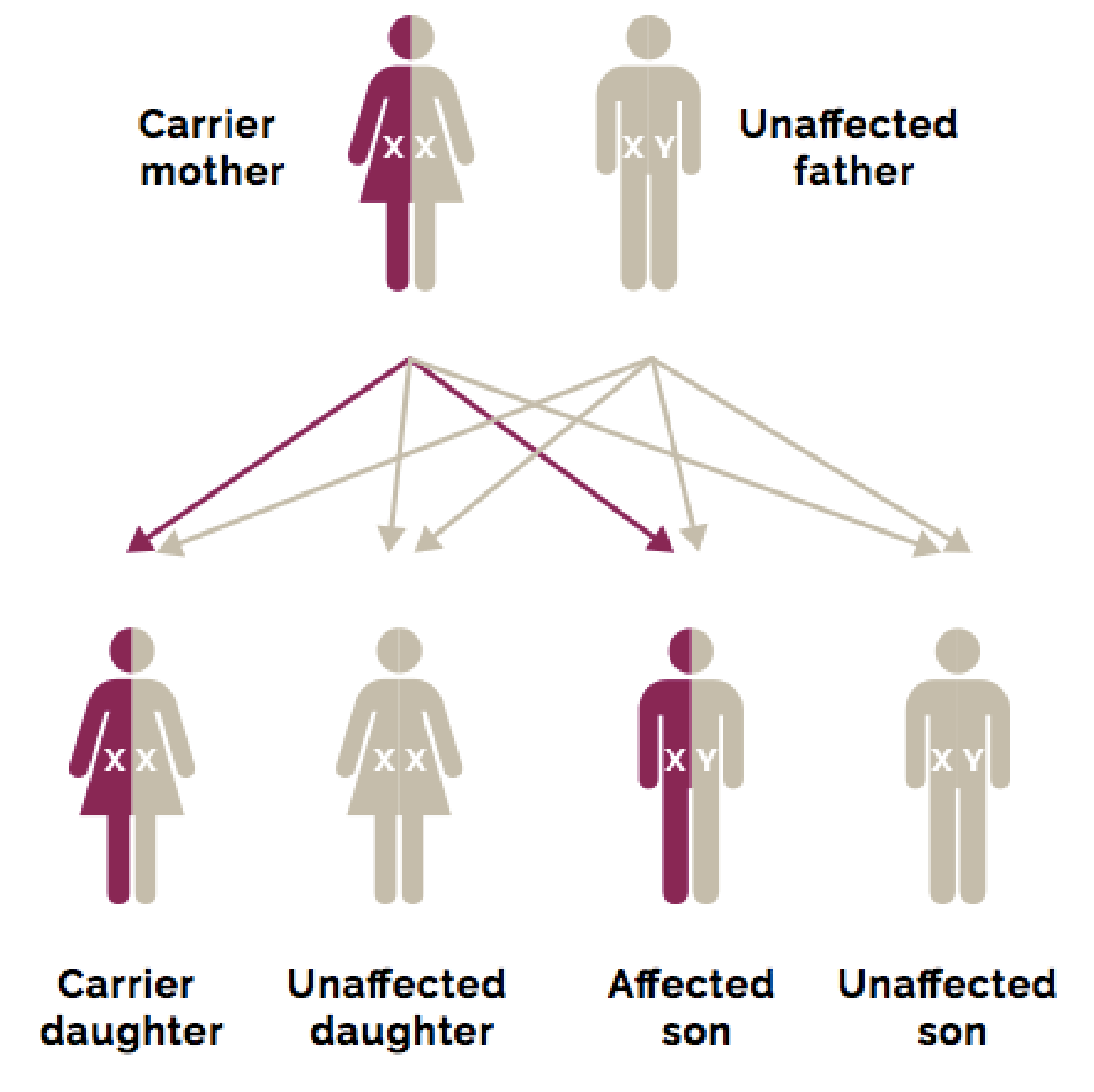
Inheritance of DMD Genetic Mutations
DMD is an X-linked genetic disorder, with two-thirds of cases caused by inherited DMD genetic mutations that are passed down from mothers to sons. The remaining one-third of DMD cases are caused by spontaneous mutations that occur in the X chromosome.3 Mutations in the DMD gene are most commonly whole-exon deletions:4-6
- Nonsense and other small mutations accounts for 20% to 27%
- Deletions 65% to 72%
- Duplications 6% to 11%
Daughters may also inherit the mutated DMD gene on an X chromosome from their mothers, but since they have 2 X chromosomes, they will likely inherit a normal gene from their fathers and be carriers of the DMD genetic mutation.7
Genetic testing is used to confirm the presence of a DMD mutation in boys with Duchenne and in girls who are carriers.1,8
When your patients’ genetic testing results are ready, genetic counselors are invaluable resources to help explain the results to your patients and their families. Genetic counselors can also provide guidance on issues such as family planning and refer patients and caregivers to support services.1,8