Therapeutic Approaches
No cure for DMD exists at present, but therapeutic options are available that may help slow disease progression, stabilize disease, or treat associated conditions.1
Other ways to help manage the impact of the disease on your patient include nutrition management, physical therapy, speech therapy, and/or psychosocial therapy.1
Steroids
Steroids, especially corticosteroids, are the most commonly used medications for DMD. They help slow down the muscle damage and weakness caused by the disease by reducing inflammation in the muscles. They may also help the heart and lungs remain stronger longer, reduce the chance of having scoliosis, and extend the ambulatory period. However, opinions differ about the best types and doses of corticosteroids to use.1
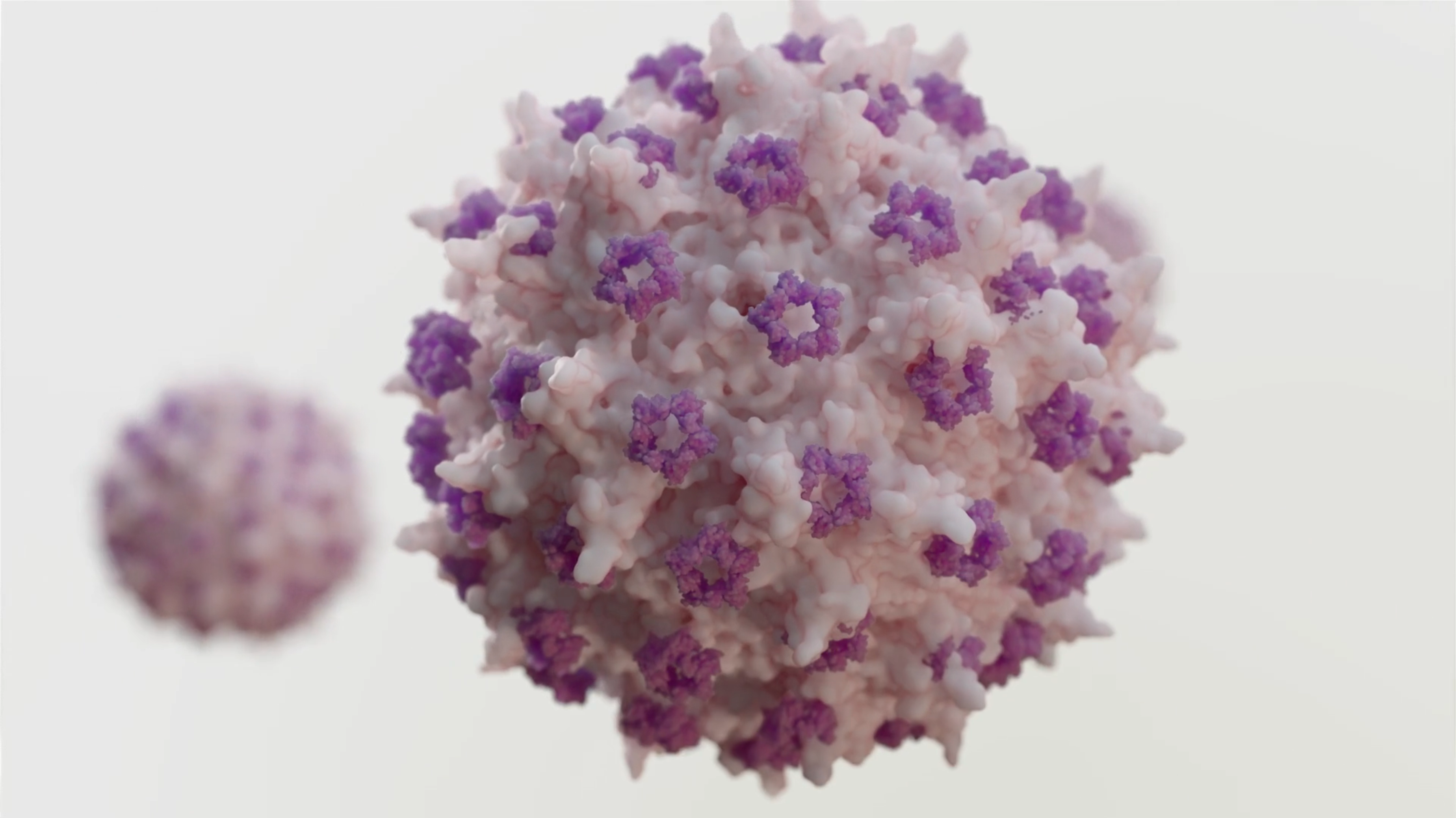
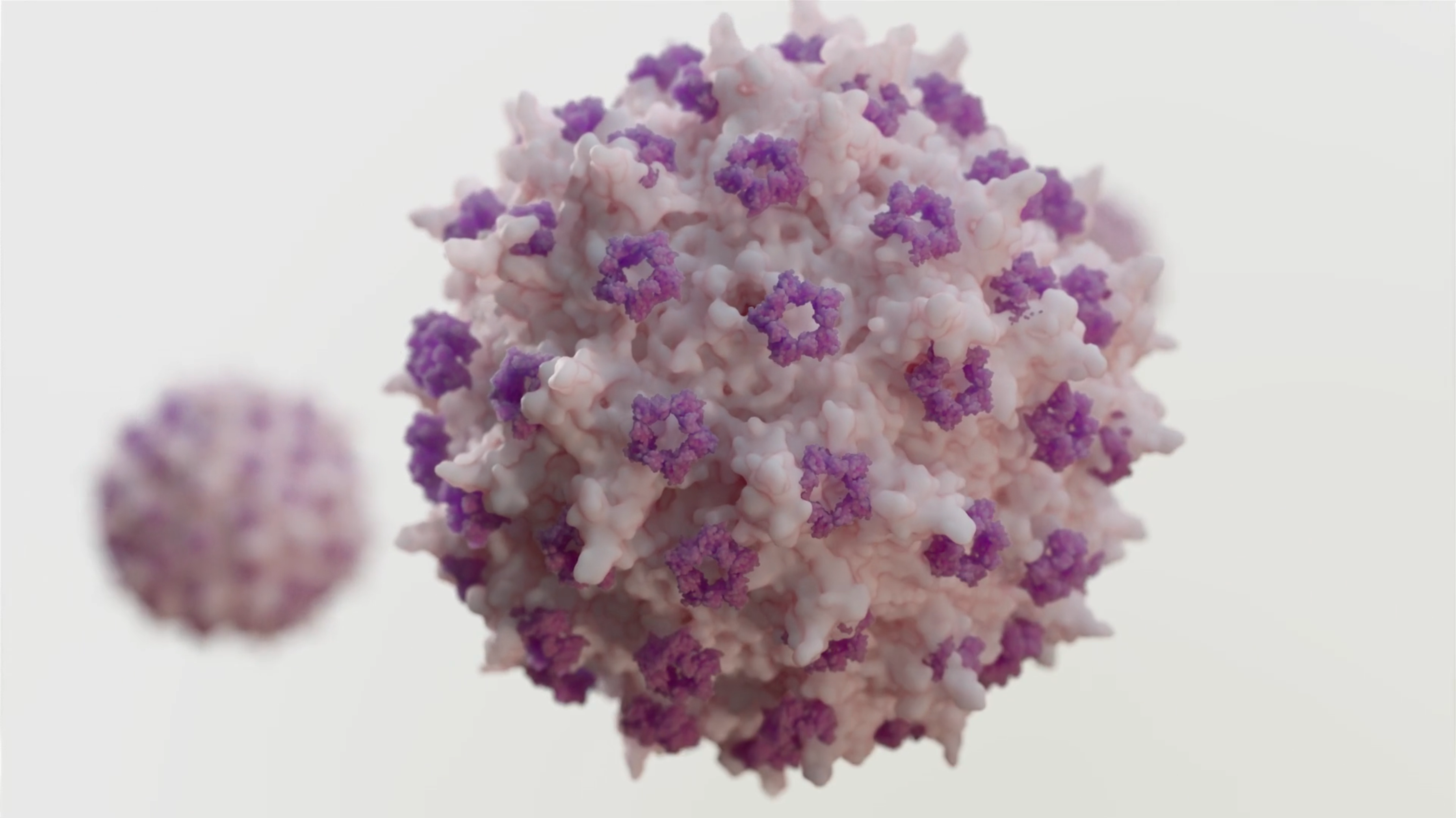
Gene Therapy
What is Gene Therapy Designed to Do?
Many neuromuscular diseases are monogenic, meaning that they are caused by mutations in a single gene that lead to muscle or motor neuron cells producing little to no working protein.2 Gene therapy is being used to treat monogenic neuromuscular diseases like DMD.3,4
The goal of gene therapy is to slow or stabilize disease by introducing a potentially functioning version of the gene into disease-affected cells.2,4
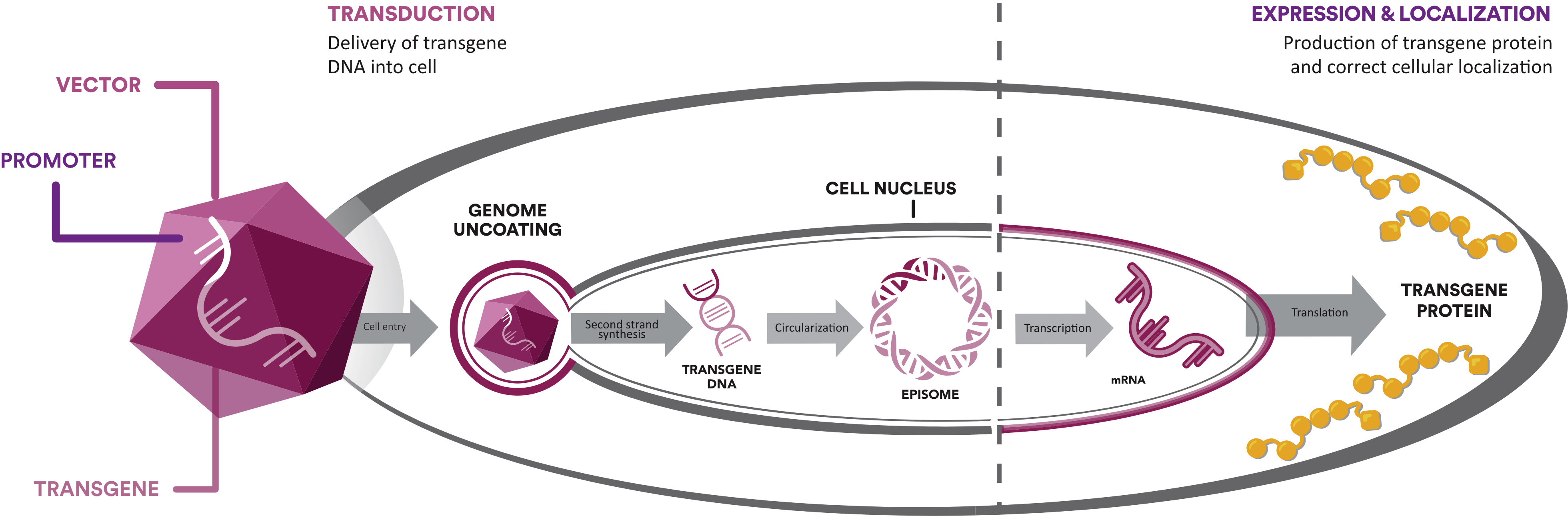
Components of Gene Therapy
Gene therapies consist of 3 core components—the vector, promoter, and transgene—with each playing an essential role.3-5

Promoter
Another component, the promoter, is a segment of DNA that works like an “on/off switch” for protein expression in the target cells.4,7,8 Different promoters have diverse patterns and strengths of expression in target cells. In designing a gene therapy, researchers select a promoter that can facilitate protein expression in target cells. For example, researchers select a promoter they believe will result in uniform protein expression across skeletal muscle cells.
Transgene
The transgene is engineered with the goal of creating a functioning version of the affected gene. Once the transgene is delivered into target cells, it is designed to direct protein expression in the cells. In DMD, the full-length dystrophin gene is too large to fit in the vector. Instead, researchers have engineered transgenes that represent a truncated version of the dystrophin gene. This transgene aims to direct protein expression of a truncated form of dystrophin in the disease-affected cells.4
Exon-Skipping Therapies
Exon-skipping technologies aim to address the underlying issue in DMD—a lack of the protein dystrophin. Many people with DMD have a genetic mutation in which one or more exons in the dystrophin gene are deleted, resulting in their cells not being able to produce dystrophin. The goal of exon skipping is to allow the body to make a shorter form of the dystrophin protein.9
A simple way to explain exon-skipping therapies to your patients and caregivers is to use the example of a train.

Normally, the DMD gene produces pre-mRNA, which contains 79 exons. Mutations in the DMD gene lead to the deletion of one or more exons in patients with DMD.10-12 Pre-mRNA splicing occurs, joining the exons together without the deleted exons.11
Think of the exons like toy train cars, each with a special connection that allows one car to connect to another. The connections between cars must match, so they can connect to one another. The connections must match up for the train to move.

Missing exons are like missing train cars. When one or more cars are missing, the connections between them no longer line up, and the train no longer functions properly. The missing exons induce an out-of-frame transcript, or frameshift. This means the body doesn't "read" the mRNA correctly, leading to structurally compromised dystrophin.10,11
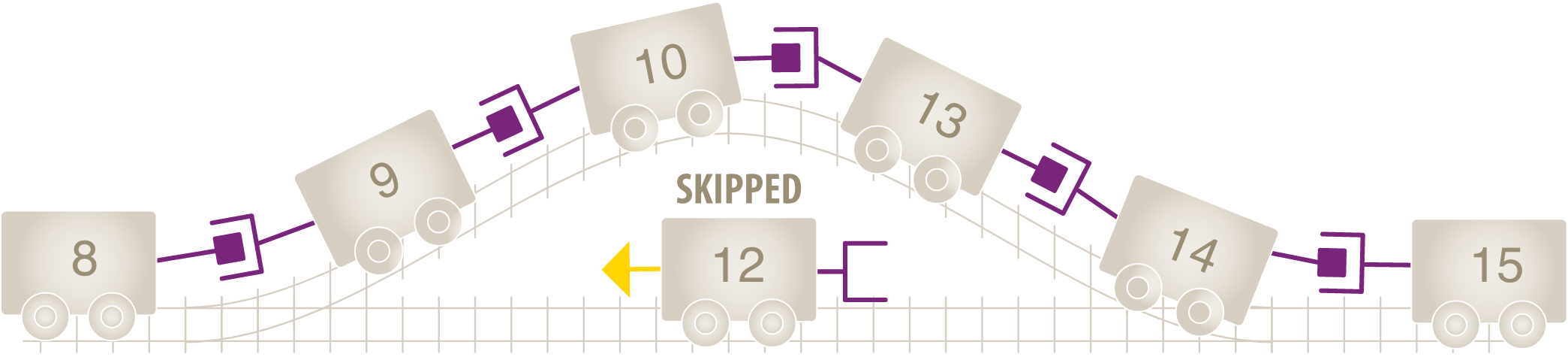
If we skip over a certain exon, or car, we can restore the connection between the remaining cars to create a shorter train.9 Skipping exon 12 creates an in-frame mature mRNA transcript that is intended to allow for the production of an internally truncated dystrophin protein. In this example, skipping exon 12 restores the reading frame of the mRNA transcript.12,13
Genetic Testing is used to determine whether someone with DMD has an exon deletion that is amenable to an exon-skipping therapy.13
Psychosocial, Cognitive, and Behavioral Therapy
Patients with DMD may also have cognitive symptoms such as speech and language delays; psychological symptoms such as anxiety, anger, and depression; and behavioral symptoms such as problems interacting with others, sitting still, or concentrating. Psychosocial and cognitive therapy, such as speech and language therapy, can help with these symptoms.14
Boys with DMD are more likely to have autism spectrum disorder (ASD) than boys without DMD.15 Symptoms of ASD include anxiety; sensory sensitivity; difficulty coping with uncertainty; poor social communication; repetitive movements and language; as well as restricted interests (strong interests in specific topics such as a particular TV show), but problems in these areas can also be due to other factors (e.g., ADHD or depression/anxiety), so it is necessary to have a careful evaluation.
If your patient has symptoms of ASD, consider referring them to a psychologist or psychiatrist. The Autism Speaks and PPMD websites are resources for patients and caregivers that discuss different types of ASD therapies, including Applied Behavior Analysis, Floortime, Treatment and Education of Autistic and Communication Handicapped Children (TEACCH), and Relationship Development Intervention. Many of these therapies are behavioral therapies that are designed to improve social and communication skills.
Day-to-Day Maintenance Physical Therapy
Physical therapy can be very helpful in maintaining flexibility, strength, and physical function.16 For example, daily stretching and the wearing of night splints or braces on the feet and ankles may help to keep joints mobile. Swim and aquatic therapy can also be helpful in maintaining joint mobility and preventing shortening and tightening of the muscles. If your patient is not ambulatory, standing devices, as well as custom seating and power positioning features on wheelchairs can also help to prevent muscle tightening.16
Managing Associated Conditions
Besides therapies that address DMD symptoms or the lack of dystrophin, patients with DMD may need treatment for heart, lung, orthopedic, and osteoporotic conditions that are often associated with DMD.17,18 This could take the form of medications, nutrition therapy, and physical and occupational therapy.